ABOUT US
Precision Multiplex
Platform Creator
Certifications and Patents
-

ISO 13485:2016
Medical Devices Quality
Management System -

CE IVDD
-

CE RoHS
-

CE EMC
-

Venture company
-

R&D Center approval
-

Frontier Venture company
-

Baby Unicorn 200
-

GMP Certification
-

QuantumPACK™ EASY RSV Ag MFDS Manufacturing approval (Class 3)
-
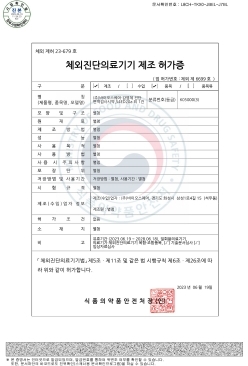
NanoPACK™ COVID-19 Ag Home MFDS Manufacturing approval (Class 3)
-
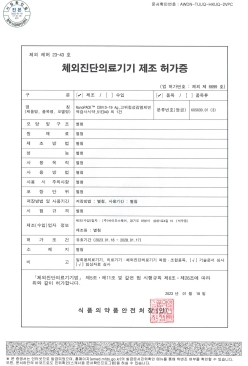
QuantumPACK™ EASY COVID-19 Ag MFDS Manufacturing approval (Class 3)
-
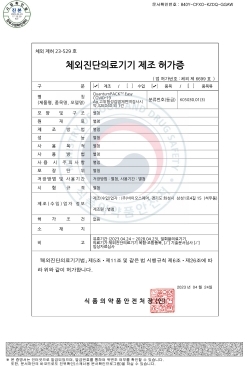
NanoPACK™ COVID-19 Ag MFDS Manufacturing approval (Class 3)
-
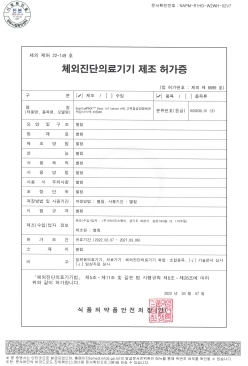
QuantumPACK™ EASY Influenza A+B MFDS Manufacturing approval (Class 3)
-
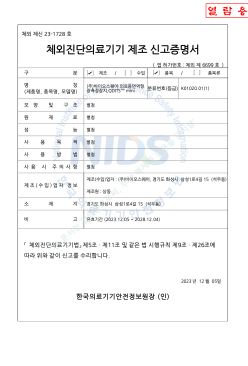
Medical Immunofluorescence Measurement Device Manufacturing Declaration (QDITS™ Mini)
-
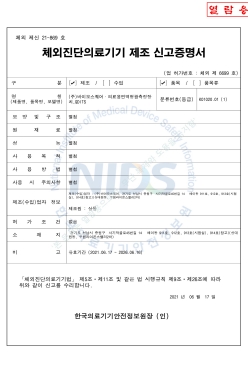
Medical Immunofluorescence Measurement Device Manufacturing Declaration (QDITS™ Basic)
-

QuantumPACK™ Improvement Patent (CN113597558)
-

QuantumPACK™ Improvement Patent (CN113614201)
-

QuantumPACK™ Improvement Patent (US 12,313,629)
-

QuantumPACK™ Improvement Patent (US11,884,852)
-

QuantumPACK™ Improvement Patent (US11,851,592)
-

QuantumPACK™ Improvement Patent (JP7353666)
-

QuantumPACK™ Improvement Patent (CN111183202)
-

QuantumPACK™ Improvement Patent (EP3666850, BE, DK, FR, GB, DE, IT, ES, SE, CH)
-

QuantumPACK™ Improvement Patent (JP7162150)
-

QuantumPACK™ Improvement Patent (JP7058401)
-

QuantumPACK™ Improvement Patent (KR102306937)
-

QuantumPACK™ Improvement Patent (KR102430149)
-

QuantumPACK™ Improvement Patent (KR102012716)
-

QuantumPACK™ Improvement Patent (KR101960616)
-

Patent exclusive license (KR1651798, QuantumPACK™)
-

Patent exclusive license (KR1609618, QuantumPACK™)